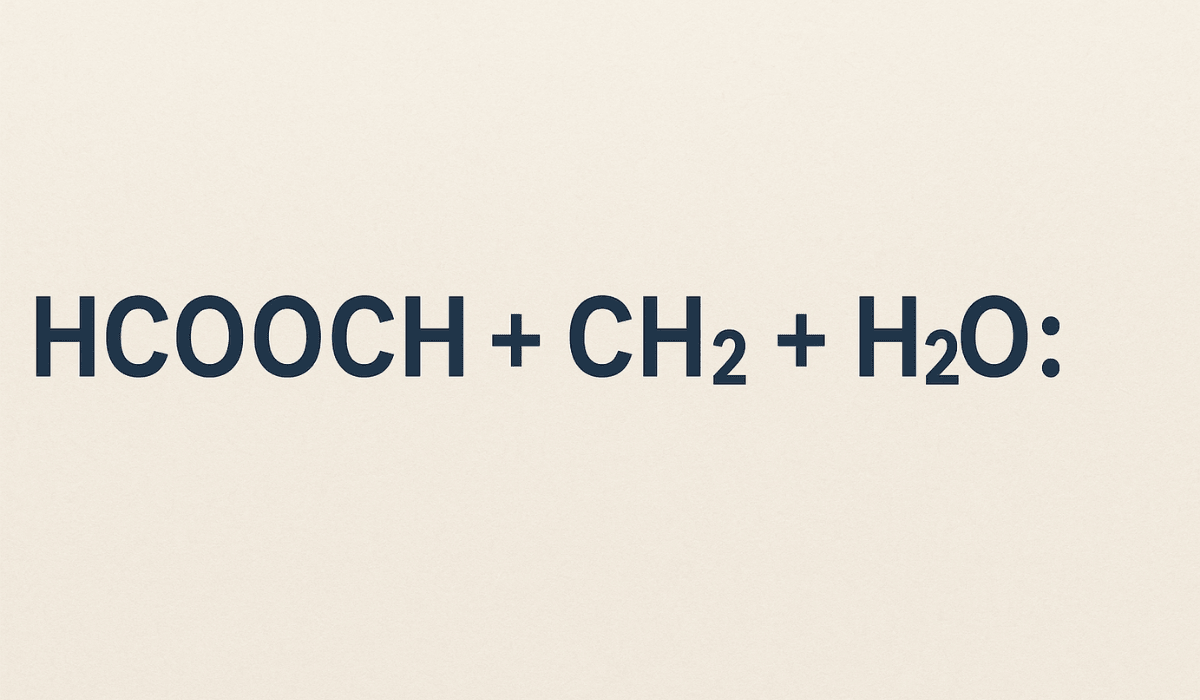A ball-and-stick model of a simple organic molecule (illustration of molecular interactions in chemistry).
Chemistry often greets us with formulas that look like secret codes. If you’ve ever come across hcooch ch2 h2o and scratched your head, you’re not alone! At first glance, it seems like random letters, but it actually hints at a small drama happening between molecules. In this article, we’ll unravel what this cryptic expression represents, explore the reaction it might describe, and see why it matters in organic and industrial chemistry. Let’s dive in with an easygoing explanation – no PhD required.
Breaking Down the Expression
What does hcooch ch2 h2o even stand for? It helps to break it into parts:
- HCOOCH – This looks like a molecular fragment. In fact, it resembles HCOOCH3, which would be the formula for methyl formate. Methyl formate is the methyl ester of formic acid (formic acid is HCOOH). In plain terms, it’s a compound made from formic acid and methanol. Formic acid is the simplest carboxylic acid (famously named after ants, Formica, because they produce it!), and an ester is what you get when an acid and an alcohol join together. So, think of HCOOCH as indicating a formic-acid-based substance – likely an ester such as methyl formate. This part of the expression is key because it contains the formyl group (HCOO–) that defines formic acid derivatives.
- CH2 – In chemistry, CH2 usually denotes a methylene group, which is a unit of one carbon and two hydrogens. This could mean a couple of things. It might represent a fragment of a molecule (for example, the –CH2– linking two parts of a larger molecule), or it could hint at a related compound like formaldehyde (CH2O) without the oxygen. In the context of our expression, CH2 suggests the presence of a single carbon unit that can connect or transform. Don’t worry – we’re not talking about some mysterious standalone “CH2” floating around, but rather a piece of an organic molecule or a reactive intermediate. It’s the part that could become a methyl group (CH3) in a reaction.
- H2O – This one you likely recognize: plain old water. Water is everywhere in chemistry – it’s the universal solvent and often a reactant in its own right. In reactions, water can play roles like hydrolysis (breaking bonds by adding water) or providing a medium for molecules to meet and react. In our expression, H2O implies that water is involved, probably to either break something apart or put something together.
Now, put those pieces back together: hcooch ch2 h2o hints at a scenario where a formic-acid-derived compound (HCOOCH…), a CH2 unit, and water are all interacting. This doesn’t correspond to one single, stable molecule but rather to a combination of substances or a reaction involving them. In simpler terms, it’s like saying “we have some formate (from formic acid), plus a methylene bit, plus water, all in the mix.”
From Ester to Acids and Alcohols: The Reaction Context
So what reaction could involve this combo? A very likely candidate is an ester hydrolysis. Let’s use the example we touched on: methyl formate and water. Methyl formate’s formula is HCOOCH3 (which our expression almost spells out), and when you add H2O to it under the right conditions, something interesting happens. They transform into formic acid (HCOOH) and methanol (CH3OH). This type of reaction is called hydrolysis – literally “splitting with water.” The water breaks the ester bond, resulting in a carboxylic acid and an alcohol.
Think of it like a molecular undoing of a handshake: formic acid and methanol once shook hands to form the ester (methyl formate); adding water makes them let go and return to their original forms. Chemists often use an acid or base as a catalyst to speed this up (for instance, a bit of hydrochloric acid or sodium hydroxide can help the reaction along). The concept is fundamental in organic chemistry – it’s how larger esters (like fats) are broken down into acids and alcohols too. (Fun fact: making soap from fats is a similar process, using water and base to break fat esters into fatty acids and glycerol. Chemistry isn’t just theory – it’s cleaning your hands too!)
To visualize the example reaction of methyl formate hydrolysis, here’s a simple comparison of what goes in and what comes out:
| Reactants (Starting Materials) | Products (After Reaction) |
|---|---|
| Methyl formate (HCOOCH3) – a simple ester | Formic acid (HCOOH) – a carboxylic acid |
| Water (H2O) – the molecule that breaks the bond | Methanol (CH3OH) – an alcohol (the other fragment) |
In the above reaction, hcooch ch2 h2o is essentially representing “methyl formate + water” → “formic acid + something with CH2 (which turned into CH3OH)”. It’s a neat little dance: the CH2 part that was once attached to the formyl group in the ester ends up as the methyl group in methanol. The formyl part becomes formic acid once again. If you try this reaction in a lab (with proper safety gear of course), you’d start with a sweet or fruity-smelling methyl formate (many esters have pleasant odors) and end up with pungent formic acid and methanol (which has a sharp alcohol smell). The change in smell is a hint that the chemistry has indeed transformed the substances – that’s real-life experience of a reaction!
Why Is This Important?
You might be thinking, “Okay, so an ester turns into an acid and an alcohol. Why do I care?” It turns out this little formula hcooch ch2 h2o touches on some big themes in chemistry and industry:
- Basic Organic Chemistry Principle: The interaction it represents (ester + water → acid + alcohol) is one of the first reactions organic chemistry students learn. It’s foundational because it shows how larger molecules can be built up or broken down. For example, the reverse of this reaction is how we form esters in the first place: esterification (combining an acid and an alcohol, producing water). In our case, formic acid and methanol would join to make methyl formate and water would be released. These back-and-forth transformations are central in biochemistry too – your body does countless water-assisted reactions to build and break molecules.
- Industrial Relevance: Formic acid and its esters pop up in manufacturing. In fact, one major method of producing formic acid at scale involves methyl formate. Factories first create methyl formate (by reacting methanol with carbon monoxide), then hydrolyze it (add water) to get formic acid and methanol back. This efficient loop is used because handling formic acid directly can be tricky, so they use an ester as an intermediate. Formic acid itself is used in textiles, leather processing, and as a preservative. Methyl formate is used as a quick-drying solvent and even as a fumigant (plus it has a pleasant odor, so it finds use in flavor and fragrance chemistry). So, the chemistry behind hcooch ch2 h2o isn’t just theoretical – it’s part of how we make everyday products.
- Green Chemistry and Energy: Formic acid is gaining attention as a green energy carrier. It can release hydrogen gas (H2) when decomposed, which could be used in hydrogen fuel cells. In such reactions, formic acid (HCOOH) splits into CO2 and H2 – effectively donating hydrogen. The combination of formic acid and a “CH2” source (like formaldehyde) also shows up in so-called reductive methylation reactions. For instance, in organic synthesis or even biochemical pathways, using formic acid alongside a compound containing a CH2 unit can add a methyl group (CH3) to another molecule. This has applications in making pharmaceuticals (where you often need to tack on a methyl group to tweak a drug’s properties). The classic example is using formic acid and formaldehyde together to convert an amine (R–NH2) into a methylated amine (R–NHCH3) – a handy trick in medicinal chemistry.
A laboratory setup with test tubes containing colored solutions, illustrating practical chemistry experiments.
In short, hcooch ch2 h2o encapsulates a scenario that is simple but powerful. It highlights how a small group of atoms can shuffle around between molecules. Whether we’re breaking apart an ester into an acid and alcohol, or using formic acid to reduce or modify other compounds, the interplay of these tiny molecules has big consequences. From an educational standpoint, it’s a great example of balancing a chemical equation and understanding conservation of matter (the atoms just rearrange – none are lost). From an applied standpoint, it’s central to processes in labs and industries around the world.
Now that we’ve covered the what and why, let’s wrap up with a quick FAQ to answer some burning questions you might still have.
FAQ
Q1: What does the formula “hcooch ch2 h2o” actually mean in plain English?
A: It’s referring to a combination of chemical components rather than a single substance. In plain English, you can read it as “a formic acid derivative (like methyl formate) interacting with a CH2 group and water.” The expression hints that an ester of formic acid (HCOOCH3, for example) is reacting with water. The result of that reaction is formic acid (HCOOH) and something containing the CH2 group (which becomes a CH3 group in an alcohol like methanol). So, it’s essentially a shorthand for a small organic reaction involving formic acid, a one-carbon fragment, and water.
Q2: Is hcooch ch2 h2o one single compound?
A: No – it’s not a single molecule with that exact formula. Instead, it represents multiple pieces. Think of it like writing an equation in words. “HCOOCH” on its own isn’t stable by itself; it needs context (like HCOOCH3 to be a real molecule). The way it’s written suggests we’re talking about an HCOO– containing compound plus something with CH2 plus water. In our discussion, that was methyl formate (HCOOCH3) and water as the reactants. So the formula is more of a mnemonic or a simplified depiction of a set of molecules/reactants rather than one unified formula. There is no single bottle on a shelf labeled “HCOOCH CH2 H2O” – but there could be one for methyl formate and one for water, which you’d mix to see the chemistry happen.
Q3: What type of reaction is involved here?
A: The key reaction we’ve been highlighting is hydrolysis – using water to break a bond. In this case, water breaks the ester bond in methyl formate, yielding formic acid and methanol. Hydrolysis is one of the fundamental reaction types in chemistry. We also touched on esterification (the reverse process, combining an acid and alcohol to form an ester and water). Additionally, formic acid can undergo redox reactions (it can be oxidized to carbon dioxide while reducing another substance). For instance, in the presence of certain catalysts, formic acid will release hydrogen – that’s a redox process. And if formaldehyde (which contains a CH2 group) is present with formic acid, you can get a reductive methylation reaction (important in some organic syntheses). But if you’re just starting out, focus on the idea of an ester breaking down with water – that’s the core story of hcooch ch2 h2o as we interpreted it.
Q4: Where might I encounter this chemistry in real life or industry?
A: Quite a few places! Industrial production of formic acid uses the chemistry of methyl formate and water. Methyl formate itself is used as a fast-evaporating solvent (for quick-dry adhesives or as a cleaning agent) and as a fumigant (for pest control in storage facilities). Formic acid is used to preserve livestock feed, tan leather, and in various cleaning products. It’s also found in nature – remember those ant stings? That’s formic acid causing the burn. In laboratories, mixtures of formic acid and formaldehyde (implicitly related to our HCOOCH + CH2 idea) are used in certain synthesis reactions, like making pharmaceuticals where a methyl group is added to a molecule. Moreover, green energy research is looking at formic acid as a way to store hydrogen for fuel cells, since it’s a liquid at room temperature and easier to handle than hydrogen gas. So, the humble components in hcooch ch2 h2o have roles from the lab bench to the factory to emerging clean energy tech. Not bad for a scrabble-looking formula, right?
Q5: Are the substances involved in “hcooch ch2 h2o” safe to handle?
A: As with any chemicals, one must be careful. Formic acid (HCOOH) is corrosive – it can cause burns on skin and its vapors are irritating to breathe. It’s a relatively weak acid compared to some others, but it’s still strong enough to sting (remember the ants!). Methyl formate is quite flammable and its vapors can be harmful if inhaled in large amounts; it also can irritate the eyes and nose. Methanol (CH3OH), one of the products we discussed, is toxic if ingested (even small amounts can cause blindness or worse) and it’s also flammable. Water, of course, is harmless in most situations, but in a reaction it can cause splattering if the mixture gets hot. The good news is that with proper lab precautions – like wearing gloves, goggles, working in a ventilated area or fume hood – chemists handle these substances routinely. In industrial settings, reactors are enclosed and have safety systems. So, while the chemistry itself is sound, one should always show respect to the reagents involved. With knowledge and caution, these chemicals can be used safely to do all the useful things we mentioned.
By now, we’ve turned the puzzling hcooch ch2 h2o into a meaningful story of molecules. From breaking apart esters to fueling future technologies, this little formula covers a lot of ground. Chemistry is full of such surprises – seemingly cryptic formulas that unfold into real-world phenomena. Hopefully, this friendly guide has demystified the code and sparked a bit of appreciation for the tiny molecular dance it represents. Happy experimenting (and observing) in the world of chemistry!